Abstract
Background
Damoctocog alfa pegol (BAY 94-9027, Jivi®) is an approved factor (F)VIII treatment indicated for use in previously treated patients with hemophilia A aged ≥12 years, based on its safety and efficacy profile as demonstrated in the PROTECT VIII trial (NCT01580293).Here, we report preliminary results of a post-marketing interventional study of damoctocog alfa pegol prophylaxis in previously FVIII-treated patients with severe hemophilia A aged ≥18 years.
Methods
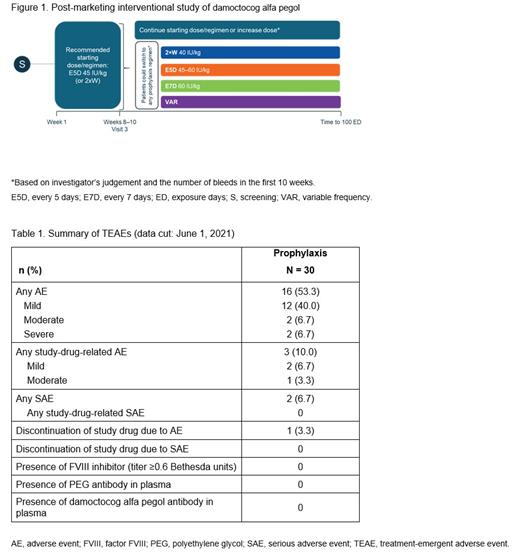
This is an ongoing, multicenter, single group, uncontrolled, open-label, post-marketing, interventional, phase IV trial (NCT04085458) of damoctocog alfa pegol in previously FVIII-treated patients with severe hemophilia A, aged ≥18 years (Figure 1). Eligible patients receive prophylaxis with damoctocog alfa pegol for 100 exposure days (EDs), starting with 45 IU/kg every 5 days (E5D) (recommended dose) or 40 IU/kg twice-weekly (2×W) until their next planned visit (Visit 3 after 10-15 EDs). At Visit 3, patients can either continue with the same regimen, increase their dose, or switch regimen to 2×W, E5D, or every 7 days (E7D), based on their needs as judged by the investigator. Patients who switched prophylaxis regimen after their third visit were assigned to a variable frequency (VAR) group. Patients with ≥1 infusion of damoctocog alfa pegol and bleeding data for ≥3 months were eligible for inclusion in the modified intent-to-treat cohort and are included in this interim analysis.
Primary endpoint is FVIII inhibitor development (titer ≥0.6 Bethesda units). Secondary endpoints include treatment emergent adverse events (TEAE), anti-PEG (polyethylene glycol) antibody development and annualized bleeding rate (ABR). Bleeds and study drug consumption were recorded using an electronic patient diary. All analyses are exploratory.
Results
By June 1, 2021, 32 patients have been enrolled. Of these, 30 patients have had ≥1 infusion of damoctocog alfa pegol and bleeding data for ≥3 months, and are thus included in this analysis (2×W, n = 7; E5D, n = 8; E7D, n = 9; VAR, n = 6). Median (range) age at start of FVIII treatment was 5.0 (0-15.0) years, with the majority (n = 24; 80.0%) starting with an on-demand regimen. Median (range) age for starting prophylaxis was 32.0 (0-64) years. At data cutoff, median (range) total time in study was 422 (112-554) days and 87 (30-114) EDs. Safety data are presented in Table 1. No patients developed FVIII inhibitors, PEG antibodies or anti-drug antibodies. Sixteen (53.3%) patients developed TEAE, with the majority being mild in severity (n = 12, 40.0%). Three (10.0%) patients experienced mild or moderate TEAEs, which were considered study drug-related by the investigator. At data cutoff, median (quartile, [Q]1; Q3) total and joint ABRs were 1.82 (0.7; 5.5) and 0.37 (0.0; 2.6), respectively. Of 20 patients who were treated for ≥12 months, 30% (n = 6) had zero total bleeds and 60% (n = 12) had zero joint bleeds in their last year of treatment.
Conclusions
Preliminary results from 30 patients observed for up to 80 weeks show that individualized prophylaxis regimens of damoctocog alfa pegol were well tolerated in this post-marketing study. No immunogenicity concerns were observed, with no patients developing FVIII inhibitors, anti-PEG or anti-drug antibodies. Despite many patients starting prophylaxis late, ABRs were low and a large proportion achieved zero bleeds. These data support the favorable safety and efficacy profile of damoctocog alfa pegol prophylaxis in routine clinical use.
Holme: Bayer, Octapharma, Pfizer and Shire: Research Funding; Bayer, Novo Nordisk, Octapharma, Pfizer, Shire and Sobi: Consultancy. Hvitfeldt Poulsen: Pfizer: Research Funding; Bayer, Novo Nordisk, Sobi, Pfizer, Octapharma: Other: Congress Support. Tueckmantel: Bayer: Current Employment. Alvarez Roman: Amgen, Bayer, CSL Behring, Novartis, Novo Nordisk, Shire/Takeda, Sobi, Roche: Speakers Bureau; Shire/Takeda: Research Funding. De Cristofaro: Shire/Takeda: Research Funding; Bayer, Takeda: Consultancy; Bayer: Other: Congress Support; Sobi: Consultancy.